Week of July 13, 2002; Vol. 162, No. 2 , p. 26
Jessica Gorman
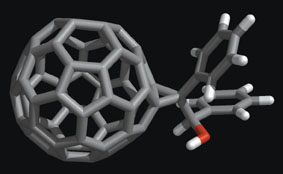
In 1991, graduate student Simon Friedman was studying drug design at the University of California in San Francisco. One day, he was chatting with Diana Roe, a fellow student, about one of the field's latest rages—HIV protease inhibitors designed to combat the AIDS virus—and the discussion turned to unexpected new therapies that might come from medicinal chemists. Suddenly, Roe exclaimed, "What are they going to try next? Buckyballs?" Buckyballs—microscopic, soccer ball-shaped cages made of exactly 60 carbon atoms—had been recognized just 5 years earlier. The Nobel prize was awarded in 1996 to their discoverers, who had formally named the molecule buckminsterfullerene for its resemblance to the geodesic domes of architect R. Buckminster Fuller. Although these molecules resembled nothing found in any pharmacy, Friedman's mind started calculating after his friend mentioned them. A buckyball, he mused, might just be exactly the right size to block the active site on the HIV protease enzyme—like a cork in a crazy-shaped wine bottle. HIV requires the protease's active site to build new copies of itself. On his computer, Friedman soon modeled the interaction of a buckyball with the HIV protease and suddenly Roe's casual suggestion seemed profound. More than a decade after Friedman and others first pondered the idea, research toward medical uses for buckyballs continues trekking forward. Buckyballs are members of a class of all-carbon, cage-shaped molecules now known as fullerenes. In recent months, for example, daylong sessions at national meetings of both the American Chemical Society and the Electrochemical Society were devoted to the topic, and at least three companies are working toward medical uses of fullerenes. Friedman notes that fullerenes' unique qualities have promise for certain types of drug design. Their small size, spherical shape, and hollow interior all provide therapeutic opportunities. Moreover, a cage of 60 carbon atoms has 60 places at which to attach chemical groups in almost any configuration. Such opportunity has led to the development of not only drug candidates for treating diseases including HIV, cancer, and neurological conditions, but also new diagnostic tools. Among these are contrast agents for X-ray and magnetic resonance imaging (see box, below).
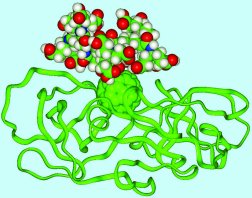
Molecular pincushion One of the best ways to use fullerenes' unique structures is as scaffolding for building drug molecules, says Friedman, now at the University of Missouri in Kansas City. "You can think of the fullerene as a molecular pincushion," agrees Uri Sagman of C Sixty, a small, Toronto company specializing in developing fullerenes for biomedical uses. A buckyball is akin to a benzene molecule, a hexagonal ring of carbon atoms used widely to make pharmaceuticals, says Sagman. Benzene can be tailored with various chemical appendages, but it's planar and floppy, so the added chemical groups sometimes interfere with one another, he says. Most drug molecules are, like benzene-based pharmaceuticals, flexible in solution. So, it's difficult to build a molecule with the precision needed to dictate intimate interactions with a target molecule, such as a protein on a cell surface, says Sagman. Because a buckyball is rigid, researchers can decorate it with clusters of atoms at specified angles and distances from one another, features that hold steady as they match up with a target. For example, chemical groups have been added to one side of a buckyball that make the molecule soluble in water while the other side of the fullerene interacts with a biological target. New York University chemist Stephen Wilson, who does research for C Sixty, has used the fullerene pincushion as a support for a variety of chemical groups with many different configurations. This effort has led to libraries of new buckyball-based molecules that the company plans to test for potential therapeutic value. Working independently, Friedman takes a different approach. He carefully synthesizes only those carbon-60 variants that his modeling and theoretical calculations suggest will be valuable. By adding this or that chemical group to specific locations on a carbon-60 scaffolding, Friedman has designed HIV protease inhibitors that bind to the protease's active site 50 times as readily as the molecules he considered in the early 1990s did. Taking a break from HIV work while waiting for further funding, Friedman is now aiming newly developed fullerenes at other biological targets. One misconception some people have is that a fullerene's many carbons, make it is very large for a drug, says Wilson. It's not—a carbon-60 fullerene is only one nanometer in diameter, roughly the size of many small pharmaceutical molecules, including Prozac and Tagamet. In comparison, a human hair is as wide as 50,000 buckyballs, he says.
Drugs in the pipeline
Research groups worldwide are developing fullerene drug candidates for a variety of diseases and testing them in animals. C Sixty reports that some of these candidates have moved well beyond the chemistry phase of drug development and that it plans to conduct human trials of fullerene-based molecules in about a year for two diseases. The company first expects to begin these trials on a modified fullerene that combats HIV. In lab and animal studies, the protease inhibitor that C Sixty plans to test on patients has proven both nontoxic and effective against several strains of HIV, says Sagman. This fullerene-based drug candidate fits snugly into the site, like a baseball into a mitt, he says. C Sixty plans to conduct its next set of human trials against amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease. In this case, the fullerene acts as a neuroprotectant—a drug that prevents or repairs neurological damage. Molecules built from the buckyball framework are efficient at eliminating so-called free radicals from cells. Free radicals appear to damage neurons in diseases such as ALS and Parkinson's disease. In rat studies, one of the fullerene-based molecules that C Sixty is developing has both stopped the degeneration of nerves and caused some motor activity to return, says Wilson. For neurological diseases—particularly ALS, which has no effective treatment and can progress rapidly—there's "an urgent health need" for new drugs, says Laura Dugan of Washington University in St. Louis. Dugan and her colleagues created and are now testing the neuroprotectant compound with funding from the National Institutes of Health. C Sixty is interested in licensing the molecule and scaling up its synthesis, she says. Rather than just absorbing the free radicals, the fullerene neuroprotectant, dubbed C3, seems to transform them into a harmless form, Dugan says. In rat studies, the potential drug has shown good results against disorders resembling Parkinson's disease or ALS and seems to be well-tolerated, she says. Dugan and her colleagues are now about to begin a study of how well the molecule works against a monkey version of Parkinson's disease. In some diseases, notably Parkinson's, part of the molecule's effectiveness might be due to its infiltration of neurons' mitochondria, cells' energy factories, Dugan says. Because mitochondria seem to play an important role in some neurodegenerative diseases, discovering that fullerene drugs get inside mitochondria "may not be a trivial finding," says Bernard Erlanger of Columbia University, who made the molecular tools that Dugan used to demonstrate the mitochondria-fullerene connection. An immunologist, Erlanger had been curious whether fullerenes injected into an animal would produce an immune response. He found that, indeed, they did. Erlanger harvested some of the antibodies that resulted in mice so that researchers could them inject then into other animals or cultured cells. Once inside such specimens, the antibodies zoom in on previously added fullerene neuroprotectant molecules. Then, the scientists added fluorescently labeled mouse antibodies that sought out the antifullerene antibodies. Dugan then located fullerenes by viewing tissue with a fluorescence microscope. A different type of antibody-fullerene duo could work therapeutically, says Lon Wilson of Rice University, who has tested a variety of potential buckyball treatments (SN: 5/8/99, p. 292). In this case, researchers would bind fullerenes to antibodies made to attach to specific cellular locations. Once there, the fullerene component would perform its medicinal duty. Alternatively, researchers have suggested, a radioactive atom might be encapsulated inside an antibody-rigged fullerene that could then carry the radiation to a target location, such as a tumor. Fullerene's future Even though they're excited by the fullerene drug candidates and diagnostic products now at various points along the development pipeline, the researchers caution that more toxicity tests in animals and various human trials are needed to prove the safety and efficacy of these newcomers to the biomedical arena. For some of the potential molecules, these tests might take a couple years; for others, much longer. Many of the proposed drugs, of course, will suffer the same fate as most conventional drug candidates do: They'll fall short of some important criterion and never make it into the pharmacopoeia. "I don't think we know all there is to know about long-term toxicity of fullerenes," comments Lon Wilson. Even so, he says, the potential benefits of using fullerenes encourage researchers to continue developing candidates suitable for clinical trials. "We're not there yet," Wilson told the attendees at the Electrochemical Society meeting. "But I expect in the next few years, we'll be hearing more talks with human clinical trials." Article archived at: http://www.sciencenews.org/articles/20020713/bob10.asp
|
Uploaded: 12-14-05