TOTAL
HEAA CONTENT IN THIS SAMPLE IS 0.000% w/w
HYBRIDIZED
CORDYCEPS SPECIES:
Plot
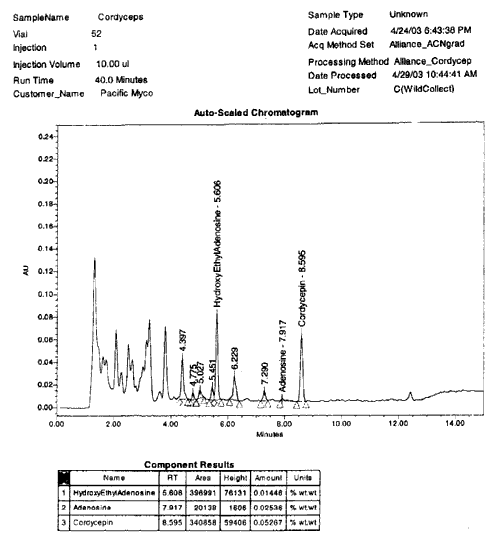
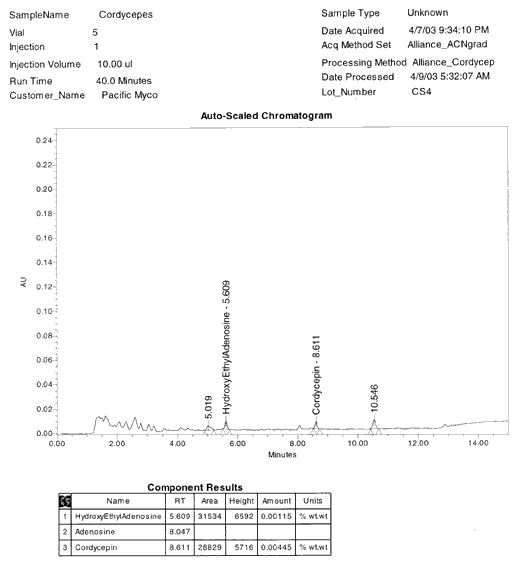
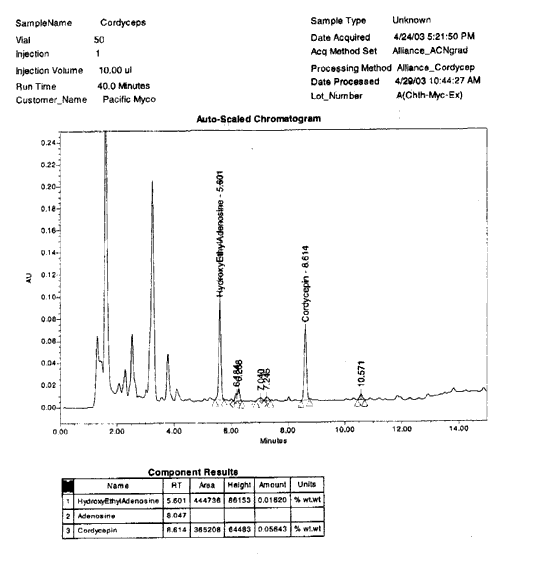
5 shows a sample of Cordyceps sinensis mycelium powder, grown
in America on
a solid substrate of grain, utilizing unique culture parameters and a specially
hybridized, non-GMO strain of Cordyceps
sinensis. This strain is detailed in section two of this paper as
the most potent Cordyceps yet known, either from the wild or cultivated.
See plot 5:
PLOT
5 – HYBRID CORDYCEPS SINENSIS ALOHAENSIS
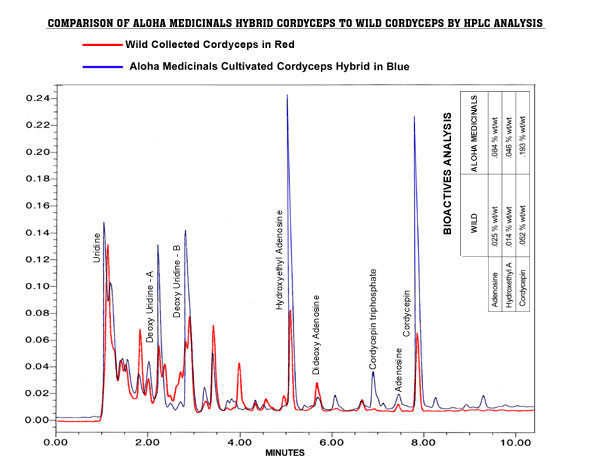
Plot
6 below is this same hybrid strain overlaid on a plot of wild Cordyceps
to show the comparison in quantity of active ingredients as well as the
qualitative similarities in the cultivated verses the wild Cordyceps.
The secondary metabolites produced are very nearly identical in these two
specimens of Cordyceps. See plot 6:
PLOT
6 – COMPARISON OF WILD TO HYBRID CORDYCEPS
PART
II – HYBRIDIZATION METHODS AND CULTURE MODIFICATION
CULTURE
METHODS AND SUBSTRATES:
In
looking at the variations in quality from different strains and producers
of Cordyceps, one must wonder what is it that causes this to be so.
After all, a tomato is a tomato, no matter where it is grown. Yet with Cordyceps,
even the same strain (CS-4) grown by different growers
turns out to be entirely different from a standpoint of active ingredients.
In looking
into this question, it is first important to realize that there are two
different methods used today in the cultivation of Cordyceps. There
is the method primarily used in China,
known as Liquid culture or Fermentation, in which the organism is introduced
into a tank of sterilized liquid medium, which has been formulated to provide
all of the necessary nutritional components for rapid growth of the mycelium.
After growth in the liquid medium, the mycelium is harvested by straining
it out of the liquid broth and drying, after which it can be used as-is
or further processed. Generally in this method the extra-cellular compounds,
which were exuded by the fungus during the growth cycle, are discarded with
the spent broth. This represents a major loss of bioactive compounds as
many of the active ingredients are extra-cellular in nature, and are found
only in small concentrations in the mycelium.
The second
cultivation method is the solid-substrate method followed by most growers
in Japan and
America. In this cultivation
system the mycelium is grown in plastic bags or glass jars full of sterilized
medium, which is almost always some type of cereal grain. This grain is
usually rice, wheat or rye although many different types of grain have
been used. After some period of growth, the mycelium is harvested along
with the residual grain. While this is an easily mastered and low capital
investment cultivation technique, the down side of this method is that
the grain content is usually greater than the mycelium content. In many
cases, the solid-substrate grown mycelium we tested was greater than 80%
residual grain. However, a bonus to this method is that the extra-cellular
compounds are harvested along with the substrate and mycelium.
Cordycepin
is an example of one of the compounds that is primarily extra-cellular
in nature. Many tests have been done on cultured Cordyceps mycelium
for the presence of Cordycepin. What is found by these tests is that in
solid-substrate grown Cordyceps, there is usually Cordycepin present,
and in liquid-cultured Cordyceps, usually none. The presence or
absence of Cordycepin is dependent upon, among other factors, by which
method the mycelium was grown and harvested.
We can see
from this that the culture method itself has an effect on the quality
of the resultant Cordyceps product. Beyond the methodology itself,
the next most important factor in the production of particular secondary
metabolites (or target medicinal compounds) is the nature and composition
of the substrate itself. (Zhang, Zhao, Wu, Bai 1992) While it would seem
that a substrate that favors rapid and strong growth of the mycelium would
be an ideal substrate to use, this is not necessarily the case. Substrates
are chosen on availability and price, or on historical usage or preference
in handling. But rarely have they been chosen on the basis of the end
compounds produced. In fact, the only way to determine whether the substrate
being used is the best choice or not, is to compare the resultant product
after growth on that particular substrate with some standard. If the end
goal of production is Cordycepin or Didioxyadenosine (or some other specific
compound) - as it is with some of the pharmaceutical companies - then
the analysis is fairly straightforward. Just look for the amount of Cordycepin
or Didioxyadenosine present and work around that. But life in the health
supplement industry is rarely so simple. First we have to assume that
we know what it is that we are looking for. Since natural products such
as Cordyceps are chemically very complex, the truth is that we
do not really know all of the components that are bioactively important.
With this
realization in mind, we set out on a mission. To produce
the best Cordyceps possible. What is the best? Since we
did not know the answer to that question, we decided to try to copy the
natural, wild collected Cordyceps as closely as possible. We attempted
this by altering the substrate composition and analyzing the resultant
mycelial product for known bioactive compounds. Then
altering the substrate again…and again…and again. We did this through
several hundred different substrates and through many thousands of kilograms
of resultant product. What we found was that there was not any single
method, strain or substrate we could use that would yield the results
desired.
SUBSTRATE:
The
substrate of choice for most Chinese growers is a liquid media based upon
silkworm residue, with added carbohydrates and minerals. This seems a logical
choice, since this mushroom is found in nature growing on insects. Dried
silkworm bodies are the by-product of an existing industry and have little
other use. Therefore they are readily available and cheap. This silkworm-based
substrate seems to yield a relatively high quality product. The only problem
with silkworm- residue based substrate is that in the United
States,
the FDA requirements are for mycelial products to be produced on a normally
consumed human food source. Silkworms do not fit into that category. They
are also not available as a raw material source to most of the worlds Cordyceps
cultivators. The
most usual substrate for Japanese and American growers is rice. It was determined
in our trials that rice is not a suitable substrate for Cordyceps
production if the target medicinal compounds are considered. Rice does not
allow the full range of secondary metabolites to be expressed by the fungus,
and rice grown Cordyceps has tested inferior in all of our analyses
of active ingredient. There is rarely any appreciable amount of Adenosine
or Cordycepin present in rice-grown Cordyceps. Furthermore, there
are growth-stunting metabolites which build up in the substrate when Cordyceps
is grown on rice, limiting the growth stage to only about 22-24 days, and
allowing no more than about 40% of the rice to be converted into mycelial
mass. This figure of 40% represents the high end of conversion, and is usually
around 25-30%. This means that when Cordyceps is grown on rice then
dried and powdered, the resultant product is actually about 60-75% rice
flour.
Rye grain
is another substrate often used for solid culture, and it yields a higher
quality product than rice, as long as a source of vegetable oil as an
amendment is added to the growth medium at the time of substrate makeup.
The oil provides necessary nutrients, which the organism utilizes for
bioactive compound production. Rye has other disadvantages though. The
compounds in rye, which give it that characteristic rye smell and taste,
are not broken down by the Cordyceps and they concentrate in the
final product. This rye taste and smell overcomes the characteristic Cordyceps
taste and smell, and even though the resultant product is of better quality
than the rice grown mycelium, there are certain perceptual problems that
needs to be overcome by the buyer to make this an economical alternative.
Rice-grown Cordyceps may seem like a better product to the average
buyer because the rice does not mask the characteristic Cordyceps
smell and taste. Most buyers in the health supplements industry tend to
purchase bulk products on perception and faith rather than requiring an
independent analysis. Rye also has growth-limiting factors, which causes
the Cordyceps growth to stunt at about 28-30 days, although this
can be overcome to a slight degree with the addition of about 1% ground
oyster shell buffer to the medium at time of make-up. We tried many other
sources of calcium, but they did not seem to work as well as the oyster
shell calcium.
Millet is
a very good choice of substrate when it is available. It has no strong
taste or smell of it’s own, it does not stunt the growth to any significant degree
and it allows for the full expression of the secondary metabolites by
the organism. It has another problem though, which is the high ratio of
chitinous outer husk layer to starch. This outer husk is not broken down
and represents a large portion of the final product weight, about 15%.
The chitinous husk cannot be removed from the grain ahead of time, since
doing so allows the grain to become too sticky during sterilization and
a high degree of anaerobic contamination follows. The husk can be removed
from the final product through mechanical means such as a time-of-flight
separation, or the product can be used for hot water extractions or other
processing. Cordyceps does not grow as fast on millet as it does
on other grains, but the end product quality is higher.
White milo
grain, also known as white kaffir corn or white sorghum is an excellent
choice of substrate. The red variety of milo does not work nearly as well
as the white variety as a substrate. White milo has all of the best characteristics;
it is cheap, it has a high starch/husk ratio, it does not stunt the growth,
it allows the full expression of bioactive compounds and has no strong
odor or taste of its own to compete with the taste and smell of the resultant
Cordyceps product. White milo when used alone however lacks some
essential ingredients required for optimum growth by the Cordyceps.
The addition of some portion of millet to the white milo speeds up the
growth by a factor of 6 times. The millet to milo ratio is optimum at
1 part millet to 4 parts white milo.
Many farmers
grow both white milo and the red milo in the same fields, or store them
in the same silos, or otherwise do not keep the white and red separated.
This is to be avoided when used as a Cordyceps substrate, since
a small proportion of the red mixed in with the white can drastically
reduce the growth rate and overall quality of the final product.
So from
our substrate testing it was determined that the ideal medium for solid
substrate growth of Cordyceps is as follows: 1 part white proso
millet (husk on) to 4 parts of white milo (husk on), with the addition
of 0.8% w/w of ground oyster shell and 1% w/w vegetable oil (peanut oil
or soybean oil). Add water to equal 50% total moisture in the sterilized
substrate. Precooking the grain mixture for 4-6 hours prior to sterilization
tends to trigger a much faster growth response from the Cordyceps.
On this medium, Cordyceps can be grown for long periods of time,
allowing nearly complete conversion of the substrate to mycelium and the
full expression of secondary metabolites from the Cordyceps. The
resultant Cordyceps when grown on this substrate is about 3-4%
residual grain, or about 96-98% pure mycelium. The real benefit to this
method of growing is the capture of the entire compliment of extra-cellular
metabolites produced throughout the entire growth process. With the addition
of certain growth triggering compounds to this mixture, Cordyceps sinensis
is easily induced to fruit in culture without any insect material being
present. However the formation of the fruitbody on this medium does not
result in any significant change to the analytical chemistry profile.
CULTURE
PARAMETER MODIFICATION: LOW TEMPERATURE HYPOXIA
Using
the above-described substrate, the complete chemical profile of the cultivated
Cordyceps still will not approach that of the wild collected Cordyceps
unless it is grown under very specific conditions. Cordyceps sinensis
produces a relatively large amount of free Adenosine when grown at normal
atmospheric oxygen levels and room temperatures. It will also produce a
large quantity of Uridine and Guanosine. But there is very little if any
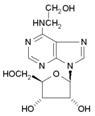
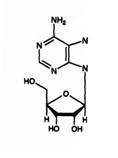
Cordycepin produced, and virtually no Hydroxyethyl Adenosine. For the organism
to produce these compounds, it needs to be growth-stressed through the absence
of oxygen, a drop in temperature and the total absence of light. Just growing
it under cold and anaerobic conditions from the start will not do the trick,
since when Cordyceps is grown under those conditions it forms a yeast-like
anamorph that has a very different chemical profile. It must first be grown
hot and fast, then tricked into converting its ‘summertime’ metabolites
into the target medicinal compounds we are looking for. To get these target
compounds, we found that we needed to follow a strict growth protocol: After
inoculation on to the millet/milo substrate, the Cordyceps is grown
at 20-22 degrees C, in diffuse light and at sea level atmospheric oxygen
for 28-30 days. It is then moved into a specially controlled environmental
chamber, where the oxygen is dropped to 50% atmospheric. The remainder of
the growth atmosphere is made up of Nitrogen, Carbon monoxide and Carbon
dioxide. The temperature is dropped to 3 degrees C, and all light is excluded.
It is held under these conditions for about 15-20 weeks. This results in
much of the Adenosine being converted to Cordycepin, Dideoxy-adenosine and
Hydroxyethyl-adenosine. Many other unique nucleosides are also produced,
with a final chemical profile identically matching that of the wild Cordyceps
as can be clearly seen in Plot 6.
HYBRIDIZATION:
Once
we had developed the substrate and growth parameters to optimize the target
compounds, we started looking into the chemical profile differences from
different strains of Cordyceps sinensis. Since there were so many
strains of Cordyceps, and each strain has its own unique chemical
profile, we tested all of the strains we were able to obtain. None of the
known strains was shown to produce nearly the quantities of active ingredients
found in the wild Cordyceps. So we started experimenting with ways
to quantitatively increase the target compound production through the hybridization
of Cordyceps strains; to cross breed them in order to gain greater
production of target compounds. This was quite a challenge. Since spore
collection and separation is very time consuming and results in entirely
too much unknown variations, we felt this method would take too much time
before we had reliable results. Rather we took a novel approach. We experimented
with various ways to get different strains of the fungi to perform their
own nuclear fusion. There are several chemicals known to trigger this exchange
of genetic material between unlike cells. Nicotinic acid for instance, can
be used to create hybridized mycelium. This compound is difficult to use
and yields unreliable results. After trying several different compounds
to trigger this fusion, what we settled on was snake venom. See Illustration
1